Featured
- Get link
- X
- Other Apps
How To Calculate Equilibrium Constants
How To Calculate Equilibrium Constants. The equilibrium constant for a reaction is calculated from the equilibrium concentrations (or pressures) of its reactants and products. The equilibrium constant is equal to the rate constant of the forward reaction divided by the rate constant of the reverse reaction.
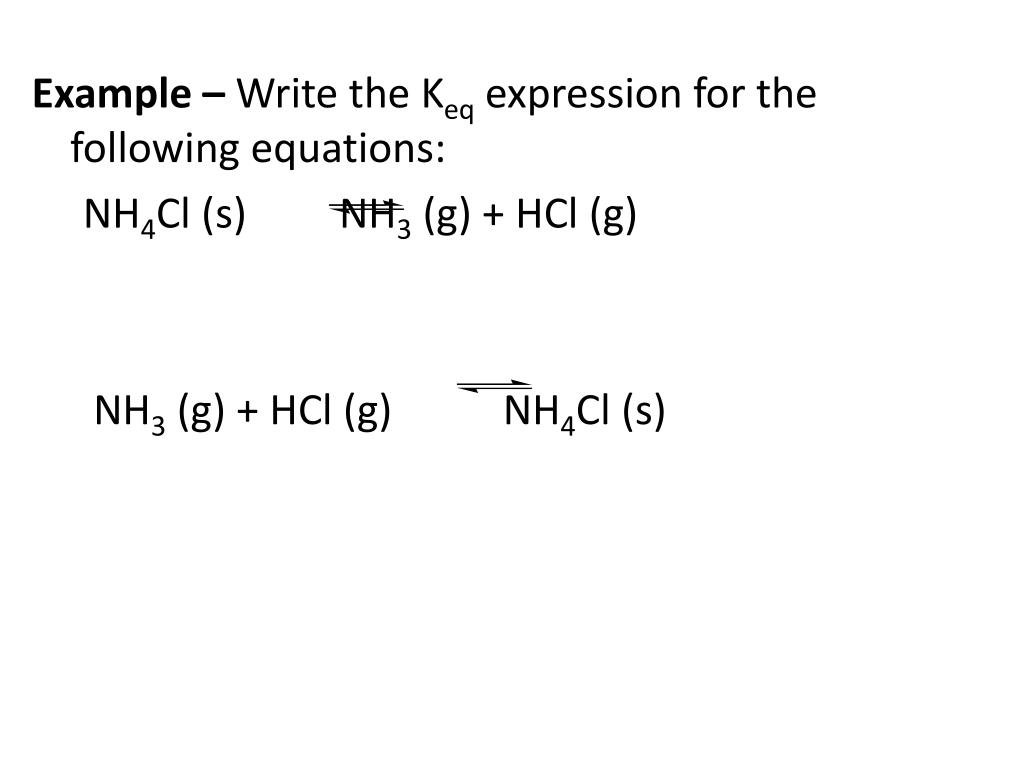
The steps are as below. To use the equilibrium constant calculator, follow these steps: When calculations involving the equilibrium constant are done, the following tips may help:
There Are A Few Steps That Need To Be Carried Out To Find The Equilibrium Concentration Of A Chemical Reaction.
E° cell = +1.13 v. The first step is to write down the balanced. E° cell = e° ox + e° red.
All Answers (5) The Ke Constant In Gibbs Free Energy Is A Dimensionless Parameter.
The value is derived using the concentrations of reactants and products in a reaction at an equilibrium state. An expression for chemical equilibrium can be written:. Calculate the equilibrium concentration of all species if 1 mol of cocl 2 is placed in a 10 l container and undergoes the following reaction.
The Steps Are As Below.
All reactant and product concentrations are constant at equilibrium. Kc is a type of equilibrium constant that links the concentration of reactant and the concentration of product in a mixture at equilibrium. Calculate the concentrations of the reactants and products step 2:
Equilibrium Is When The Rate Of The Forward Reaction Equals The Rate Of The Reverse Reaction.
That means that all the powers in the equilibrium constant expression are 1. The equilibrium constant for a reaction is calculated from the equilibrium concentrations (or pressures) of its reactants and products. Estimate the value of the equilibrium constant at 655.
You Don't Need To Write Those Into The K C Expression.
Ln (k eq) = a' + b'·ln (t/k) + c'/t. Write out the balanced chemical equation with the concentrations of beneath each substance step 3: For a chemical reaction, the equilibrium constant can be defined as the ratio between the amount of reactant and the amount of product which is used to determine chemical behaviour.
Comments
Post a Comment